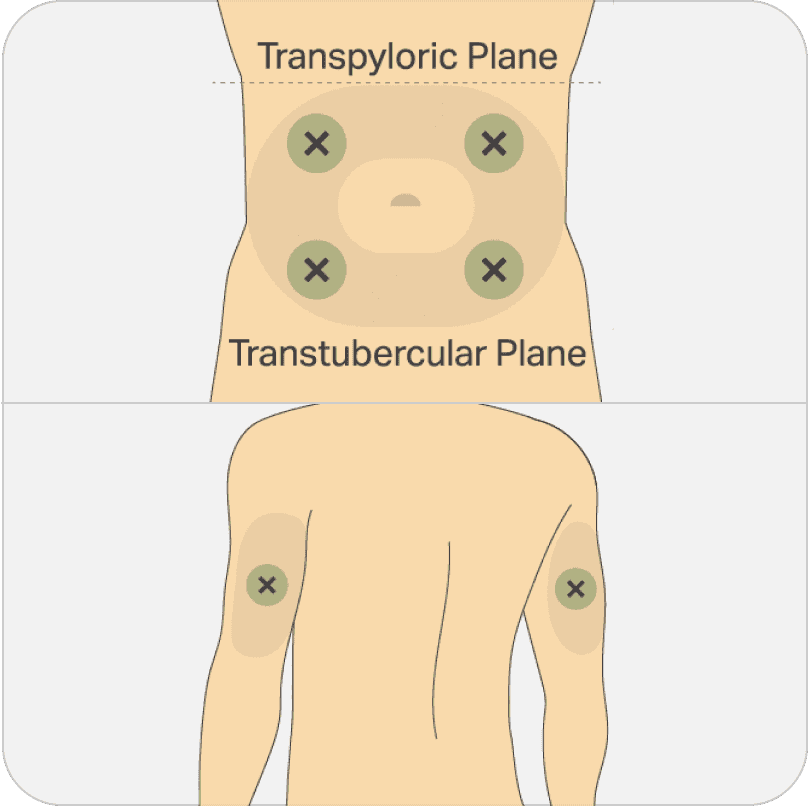
PERSERIS is injected into the subcutaneous tissue in the abdomen or back of upper arm.
Do not administer by any other route.
Must be administered by a healthcare provider.

PERSERIS® is indicated for the
treatment of schizophrenia in adults.
PERSERIS is the first-in-market risperidone-containing LAI administered in Indivior's proprietary risperidone gel depot delivery system.
LAI=long-acting injectable.
PERSERIS is injected into the subcutaneous tissue in the abdomen or back of upper arm.
Do not administer by any other route.
Must be administered by a healthcare provider.
A risperidone-containing depot is formed on contact with tissue fluids.
Risperidone is released on initial depot formation, followed by sustained drug release from the depot over the entire 1-month dosing period.
PERSERIS is injected into the subcutaneous tissue in the abdomen or back of upper arm.
Do not administer by any other route.
Must be administered by a healthcare provider.
A risperidone-containing depot is formed on contact with tissue fluids.
Risperidone is released on initial depot formation, followed by sustained drug release from the depot over the entire 1-month dosing period.
The color and distribution of risperidone are for illustrative purposes only.
For dosing and administration instructions, please see Section 2 of the full Prescribing Information.
A direct correlation between the delivery system and symptom improvement has not been clinically evaluated.
1
1
Find adequate subcutaneous tissue in the abdomen or back of upper arm free of skin conditions such as nodules, lesions or excessive pigment. Clean the injection site well with an alcohol pad.
To help minimize irritation, rotate injection sites with each monthly injection. Do not inject into an area where the skin is irritated, reddened, bruised, infected or scarred.
2
2
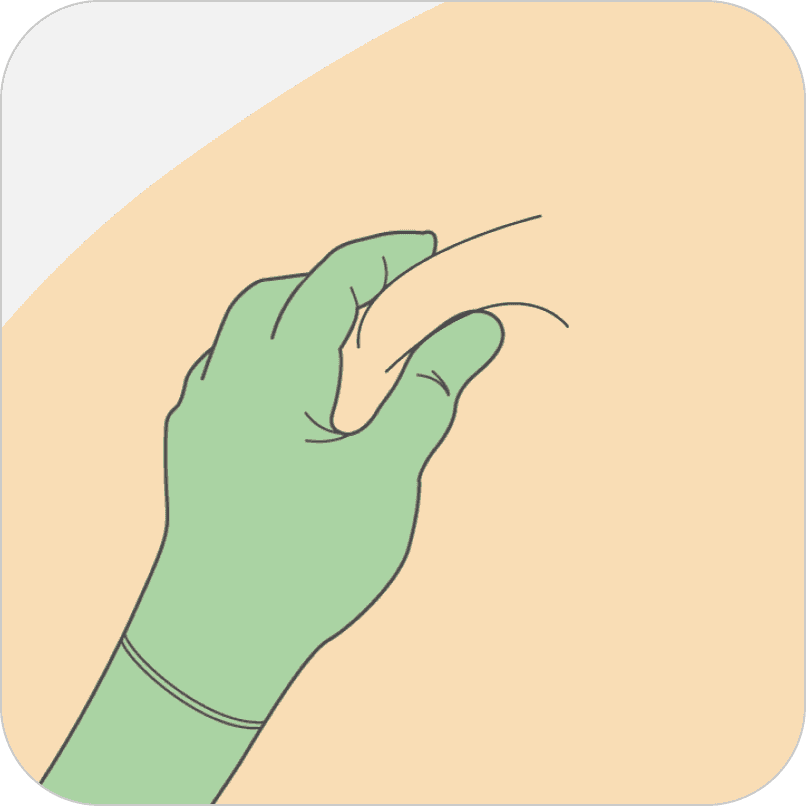
Pinch the skin around the injection area. Be sure to pinch enough skin to accommodate the size of the needle.
Lift the adipose tissue from the underlying muscle to prevent accidental intramuscular injection.
3
3
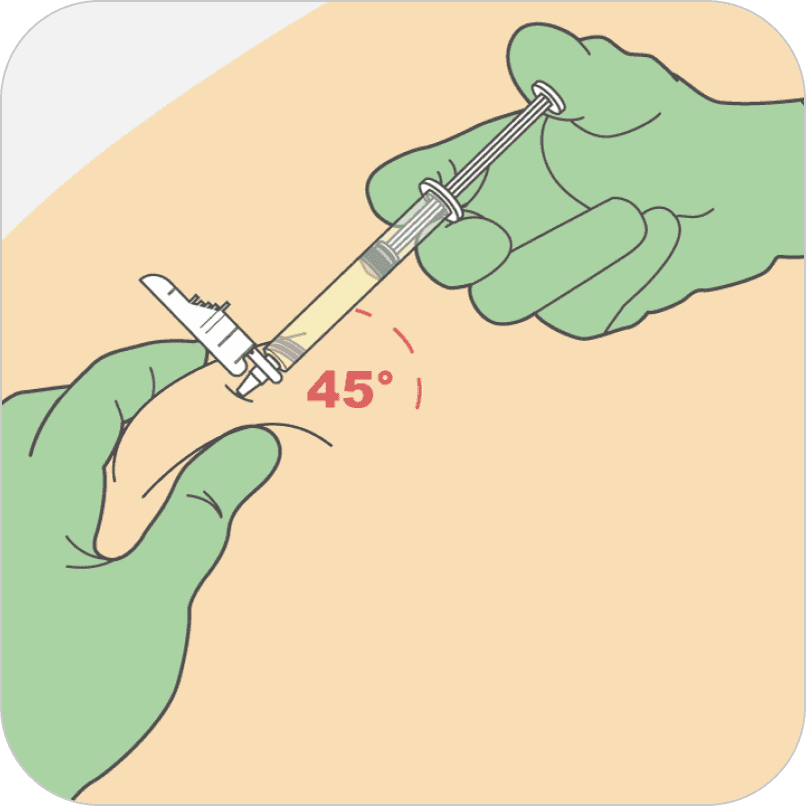
Insert needle fully into the subcutaneous tissue. Inject the medication slow and steady. PERSERIS is for subcutaneous administration only. Do not inject by any other route.
NOTE: Recommended injection angle is 45 degrees, but the actual angle of injection will depend on the amount of subcutaneous tissue.
4
4
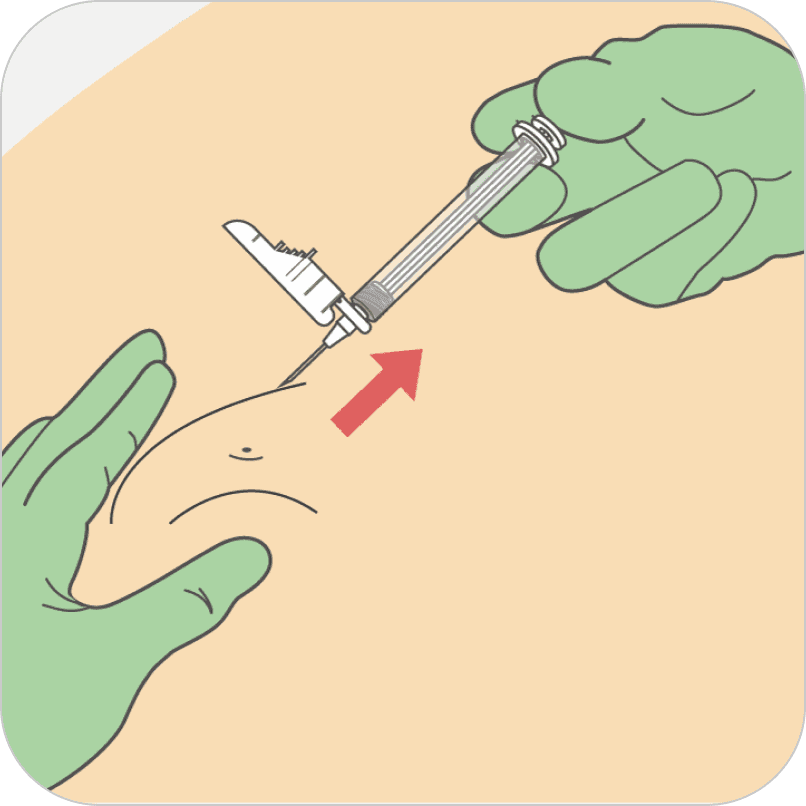
Withdraw at the same angle used for insertion and release pinched skin.
Do not rub the injection area after the injection. If there is bleeding, apply a gauze pad or bandage, but use minimal pressure.
Take the steps in the video below to start your patients on PERSERIS.
PERSERIS® (risperidone) for extended-release injectable suspension, for subcutaneous use, 90 mg and 120 mg.
INDICATION: PERSERIS is indicated for the treatment of schizophrenia in adults.
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. PERSERIS is not approved for the treatment of patients with dementia-related psychosis.
CONTRAINDICATIONS: PERSERIS is contraindicated in patients with a known hypersensitivity to risperidone, its metabolite, paliperidone, or to any of its components. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone or paliperidone.
Additional Important Safety Information is provided throughout this video transcript.
PERSERIS Instructions for Use Video
Desiree Matthews, Patient Actor, Voiceover
Desiree Matthews:
Today, I get the opportunity to cover a really salient topic for our practices, which is preparation and administration of a long-acting injectable treatment for adults with schizophrenia. My name is Desiree Matthews, and I have worked in community mental health since 2016 as a psychiatric mental health nurse practitioner. Much of that time has been spent working with individuals living with schizophrenia. I am being compensated for my time by Indivior.
Voiceover Talent:
Preparation and Administration Instructions for PERSERIS.PERSERIS is indicated for the treatment of schizophrenia in adults. The PERSERIS prescribing information includes a boxed warning regarding an increased risk of death in elderly patients with dementia-related psychosis who are treated with antipsychotic drugs. Also note that PERSERIS isn’t approved in patients with dementia-related psychosis. PERSERIS is contraindicated when there is a known hypersensitivity to risperidone or its metabolite, paliperidone, or any of the components. Hypersensitivity reactions include anaphylactic reactions and angioedema. Both have been reported with risperidone as well as paliperidone. Please see additional Safety Information and full prescribing information, including BOXED WARNING, accompanying this presentation and at www.PERSERISHCP.com.
Desiree Matthews:
Hello again! Now I would like to demonstrate the appropriate preparation and administration of PERSERIS. We will also show options for appropriate subcutaneous injection sites and provide direction to additional resources for information not covered in this video. Next we will look at key points in the PERSERIS Instructions for Use.
PERSERIS must be administered by a healthcare provider. PERSERIS should be administered only by subcutaneous injection in the abdomen or back of the upper arm. It should not be administered by any other route. Only prepare the medication when you are ready to administer the dose. As a universal precaution, always wear gloves before giving an injection. PERSERIS should be refrigerated at 36 to 46 degrees Fahrenheit. The PERSERIS kit should be brought to room temperature and kept at room temperature for at least 15 minutes before mixing. PERSERIS can remain at room temperature for up to 30 days in its original packaging. After 30 days, it should be discarded appropriately. For complete information on how to administer PERSERIS in the abdomen or back of the upper arm, please refer to Section 2.4, Preparation and Administration Instructions, of the full Prescribing Information, or visit www.PERSERISHCP.com. Now, let’s look at how to prepare PERSERIS for use. I always have my supplies laid out ahead of time, such as my sharps container, gloves, alcohol prep pads, and the medication.
To prepare PERSERIS for use, first ensure that you have one Liquid Syringe prefilled with the delivery system, one Powder Syringe prefilled with risperidone powder, and one sterile 18-gauge, 5/8-inch safety needle. Then inspect the liquid solution for foreign particles. The solution should be colorless to yellow. The syringe containing the liquid solution is the syringe that will be used to administer the injection. Now inspect the Powder Syringe for consistency of powder color and foreign particles. The powder should be white to yellow.
Hold the Powder Syringe upright and tap the barrel of the syringe to dislodge the packed powder. The powder can become packed during shipping.
Remove the cap from the Liquid Syringe. Remove the cap from the Powder Syringe. Holding both syringes in your nondominant hand can help with this step.
Place the Liquid Syringe on top of the Powder Syringe to prevent powder spillage. Connect the syringes by twisting approximately three-quarters of a turn. Do not overtighten as the syringes are being twisted. Keep your fingers off the plungers during this step to avoid spillage of the medication.
For premixing, you will need to transfer the contents of the Liquid Syringe into the Powder Syringe. Gently push the Powder Syringe plunger until you feel resistance, in order to wet the powder and avoid compacting. Pushing the Liquid Syringe plunger then the Powder Syringe plunger completes one cycle. Repeat this gentle back-and-forth process for 5 cycles. Now to do the complete mixing, continue mixing the syringes for an additional 55 cycles, making the complete mixing process 60 full cycles. This mixing can be more vigorous than when premixing. After the mixing is completed, the mixture should be a cloudy suspension that is uniform in color. The color can vary from white to yellow-green in color. If you see any clear areas in the mixture, continue to mix until the distribution of the color is uniform. Failure to fully mix the medication could result in incorrect dosage.
Now, let’s prepare the syringe. First, transfer all contents into the Liquid Syringe. Then, simultaneously maintain slight pressure on the Powder Syringe plunger and pull back gently on the Liquid Syringe plunger while twisting the syringes apart. Finally, attach the safety needle by twisting until finger tight. Check that the medication is uniform in color and free of foreign particles. Failure to aspirate the liquid from the Powder Syringe may result in incorrect dosage.
Hi Hunter, it’s good to see you today. I am going to give you a few minutes to relax before I give your injection. Do you have any questions before we begin?
Patient Actor:
No ma’am.
Desiree Matthews:
A recommendation based on my clinical experience is to always acknowledge a patient’s concern if they ask “will the injection hurt” and don’t minimize their fear of needles or pain. Discuss ways that may help to manage pain.
Now that you have prepared the medication, it’s time to prepare the site of injection. For the purposes of this video, we will review how to give a subcutaneous injection in the back of the upper arm. I work with the patient to determine the best choice of administration, either the back of the upper arm or the abdomen. For information on how to administer PERSERIS in the abdomen, please refer to Section 2.4, Preparation and Administration Instructions, of the full Prescribing Information, or visit www.PERSERISHCP.com. Hunter, would you please stand up? May I touch the back of your arm?
Patient Actor:
Sure.
Desiree Matthews:
Choose an injection site with adequate subcutaneous tissue that is free of skin conditions such as nodules, lesions, and excessive pigment. Do not inject into an area where the skin is irritated, reddened, bruised, infected, or scarred in any way. Clean the injection site well with an alcohol pad. Regardless of whether you choose the back of the upper arm or abdominal administration, to help minimize irritation it is important to rotate the injection sites each dosing cycle following a pattern similar to the illustration. If you want to use the same injection site, make sure it is not the same spot on the injection site that you used the last time. Please see the illustration on screen for all subcutaneous injection site options.
Prior to injection, remove excess air from the syringe by holding the syringe upright for several seconds to allow air bubbles to rise. The medication is viscous so bubbles will not rise as quickly as they would in an aqueous solution. Once bubbles have risen to the top of the medication, remove the needle cover and slowly depress the plunger to push out excess air from the syringe. If medication is seen at the needle tip, pull back slightly on the plunger to prevent medication spillage.
To prepare for injecting the medication, pinch the skin around the injection area. Be sure to pinch enough skin to accommodate the size of the needle. Lift the adipose tissue, or fat, from the underlying muscle to prevent accidental intramuscular injection.
My clinical practice is to ask the patient to do deep breathing or engage them in conversation as a distraction when giving the injection. To inject the medication, insert the needle fully into the back of the upper arm subcutaneous tissue at about a 45- degree angle. The actual angle of injection will depend on the amount of subcutaneous tissue present. Use a slow, steady push to inject the medication and continue pushing until all the medication has been administered. PERSERIS is for subcutaneous administration only. Do not inject by any other route.
After the medication has been administered, withdraw the needle at the same angle used for insertion, and release the pinched skin. Do not rub the injection area after the injection. If there is bleeding, apply a gauze pad or bandage, but use minimal pressure.
Lock the needle back into place by pushing it against a hard surface such as a table then dispose of all syringe components in a secure sharps container.
Hunter, remember, you may have a lump for several weeks that will decrease in size over time. Don’t rub or massage the injection site and be aware of any placements of belts, waistbands, sleeves, cuffs, or other parts of clothing. Thank you for coming in today and if you have any questions or concerns when you get home, please reach out and call the office. We look forward to seeing you back in 4 weeks for your next injection and to discuss your treatment plan. Please remember that PERSERIS should only be given by a healthcare provider or a trained pharmacist certified to give long-acting injectables.
I hope this video demonstration has been helpful to illustrate the steps to prepare and administer PERSERIS. For more information about PERSERIS, see the full Prescribing Information, including BOXED WARNING, or visit www.PERSERISHCP.com.
P-RAG-US-00552 EXPIRY: September 2025
Storage & Preparation
Once-Monthly Dosing
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. PERSERIS is not approved for the treatment of patients with dementia-related psychosis.
CONTRAINDICATIONS: PERSERIS is contraindicated in patients with a known hypersensitivity to risperidone, its metabolite, paliperidone, or to any of its components. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone or paliperidone.
WARNINGS AND PRECAUTIONS
Cerebrovascular Adverse Reactions: In trials of elderly patients with dementia-related psychosis, there was a significantly higher incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities, in patients treated with oral risperidone compared to placebo. PERSERIS is not approved for use in patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported with antipsychotic medications. Clinical manifestations include hyperpyrexia, muscle rigidity, altered mental status including delirium, and autonomic instability (see full Prescribing Information). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue PERSERIS and provide symptomatic treatment and monitoring.
Tardive Dyskinesia (TD) may develop in patients treated with antipsychotic drugs. The risk of developing TD and likelihood that it will become irreversible are believed to increase with treatment duration and total cumulative dose. TD can develop after relatively brief treatment periods even at low doses, or after treatment discontinuation. Elderly patients, especially elderly women, appear to be at increased risk, but it is impossible to predict which patients will develop TD. Therefore, PERSERIS should be prescribed in a manner that is most likely to minimize the occurrence of TD. Discontinue treatment if clinically appropriate.
Metabolic Changes that may increase cardiovascular/cerebrovascular risk, have been associated with atypical antipsychotics (APs).
Hyperprolactinemia: Risperidone elevates prolactin levels, and the elevation persists during chronic administration. Risperidone is associated with higher levels of prolactin elevation than other antipsychotic agents. Hyperprolactinemia may inhibit reproductive function in female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating drugs. Long-standing hyperprolactinemia, when associated with hypogonadism, may lead to decreased bone density in females and males.
Orthostatic Hypotension and Syncope: Risperidone may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope, particularly at treatment initiation, re-initiation, or dose increase. Use with particular caution in patients with known cardiovascular disease, cerebrovascular disease, and conditions which predispose patients to hypotension, and in the elderly and patients with renal or hepatic impairment. Monitor such patients and consider a dose reduction if hypotension occurs.
Falls: Somnolence, postural hypotension, motor instability, and sensory instability have been reported with the use of antipsychotics, including PERSERIS, which may lead to falls, and consequently, fractures or other fall-related injuries. Assess the risk of falls when initiating treatment and recurrently during treatment.
Leukopenia, Neutropenia, and Agranulocytosis have been reported with antipsychotic agents, including risperidone. In patients with history of clinically significant low white blood count (WBC) or absolute neutrophil count (ANC), or history of drug-induced leukopenia or neutropenia, perform a complete blood count frequently during the first few months of therapy. Consider discontinuation at the first sign of clinically significant decline in WBC in the absence of other causative factors. Monitor patients with clinically significant neutropenia for fever or other symptoms/signs of infection; treat promptly if such symptoms/signs occur. Discontinue PERSERIS in patients with ANC <1000/mm3 and follow WBC until recovery.
Potential for Cognitive and Motor Impairment: Antipsychotics including PERSERIS may cause somnolence and impair judgment, thinking, and motor skills. Caution patients about operating machinery, including motor vehicles, until they are reasonably certain PERSERIS does not affect them adversely.
Seizures were observed in risperidone studies in adults with schizophrenia. PERSERIS should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold.
Dysphagia: Esophageal dysmotility and aspiration can occur. Use cautiously in patients at risk for aspiration.
Priapism has been reported with other risperidone products. Severe priapism may require surgical intervention.
Body Temperature Regulation: Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature. Use with caution during strenuous exercise, exposure to extreme heat, dehydration, or when taking anticholinergic medications.
ADVERSE REACTIONS: The most common adverse reactions in a clinical trial (≥ 5% and greater than placebo) were increased weight, constipation, sedation/somnolence, pain in extremity, back pain, akathisia, anxiety, and musculoskeletal pain. The most common injection site reactions (≥ 5%) were injection site pain and erythema. This is not a complete list of potential adverse events. Please see the full Prescribing Information for a complete list.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: PERSERIS may cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. Advise patients to notify their healthcare professional if they become or intend to become pregnant during treatment with PERSERIS. Patients exposed to PERSERIS during pregnancy may be registered with the National Pregnancy Registry for Atypical Antipsychotics (1-866-961-2388 or http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/).
Lactation: Infants exposed to risperidone through breastmilk should be monitored for excess sedation, failure to thrive, jitteriness, and extrapyramidal symptoms.
Pediatric Use: Safety and effectiveness of PERSERIS have not been established in pediatric patients.
Renal or Hepatic Impairment: Carefully titrate on oral risperidone up to at least 3 mg before initiating treatment with PERSERIS at a dose of 90 mg.
Patients with Parkinson’s Disease or dementia with Lewy Bodies can experience increased sensitivity to risperidone. Manifestations can include confusion, obtundation, postural instability with frequent falls, extrapyramidal symptoms, and clinical features consistent with NMS.
To report a pregnancy or side effects associated with taking PERSERIS or any safety related information, product complaint, request for medical information, or product query, please contact PatientSafetyNA@indivior.com or 1-877-782-6966.
P-RAG-US-00532